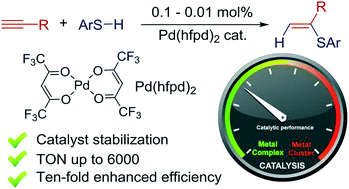
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand - Catalysis Science & Technology (RSC Publishing)

Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation | Science
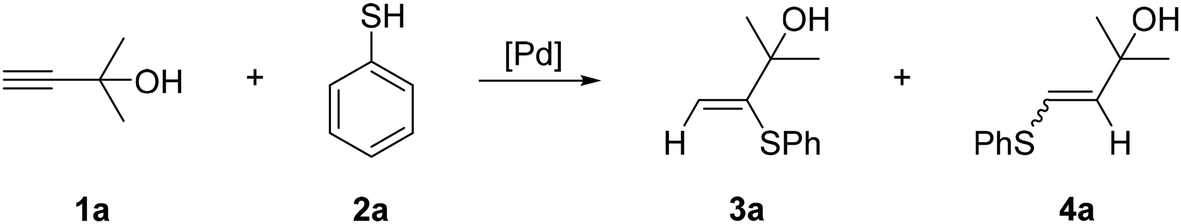
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand - Catalysis Science & Technology (RSC Publishing)
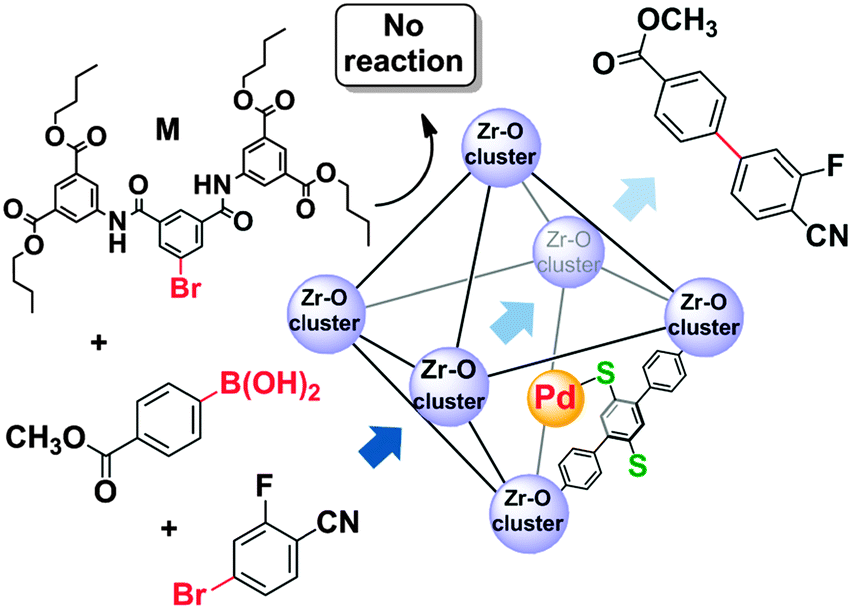
Tackling poison and leach: catalysis by dangling thiol–palladium functions within a porous metal–organic solid - Chemical Communications (RSC Publishing)

Pd/BIPHEPHOS is an Efficient Catalyst for the Pd‐Catalyzed S‐Allylation of Thiols with High n‐Selectivity - Schlatzer - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library
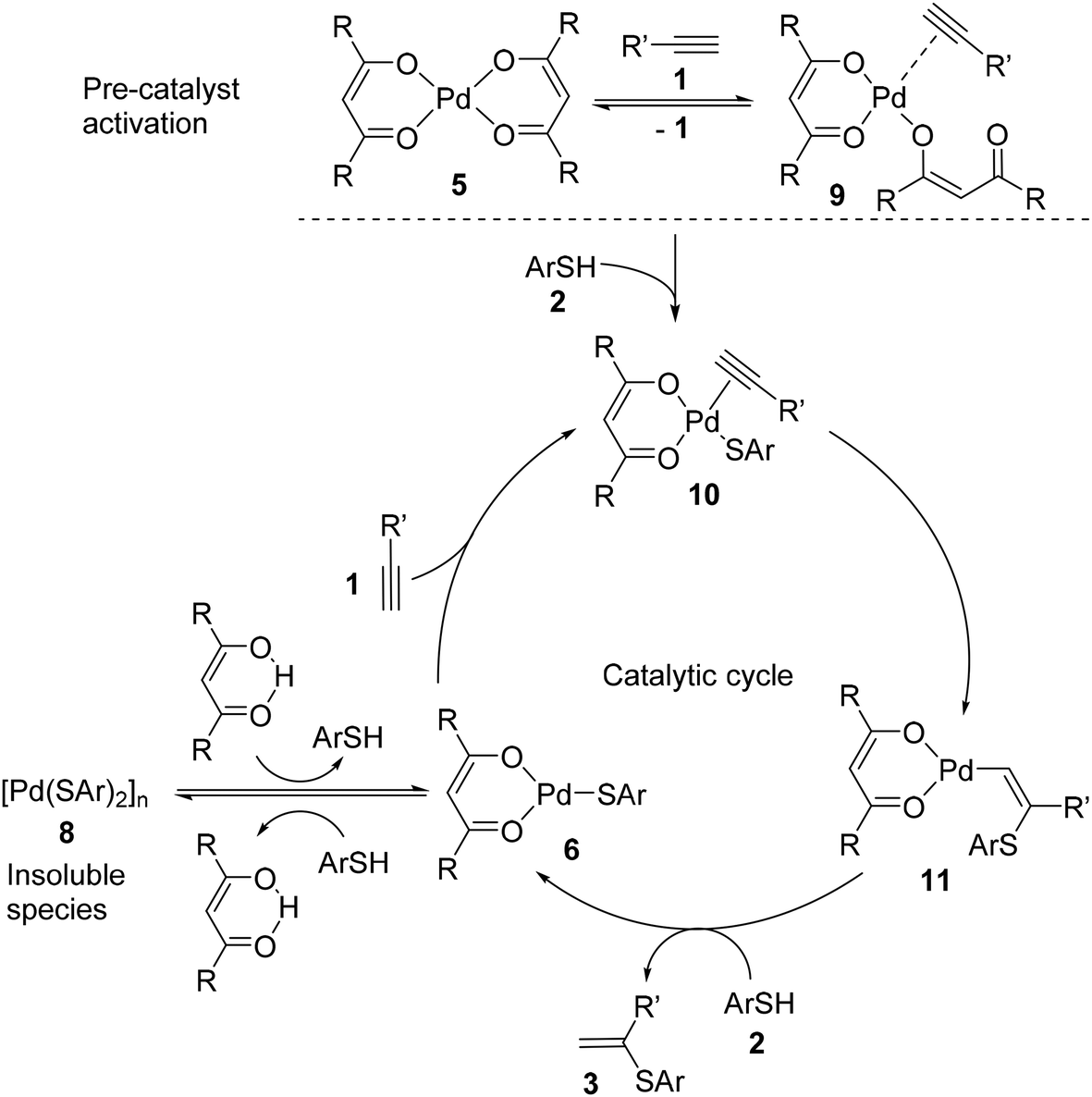
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand ... - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/C8CY00173A
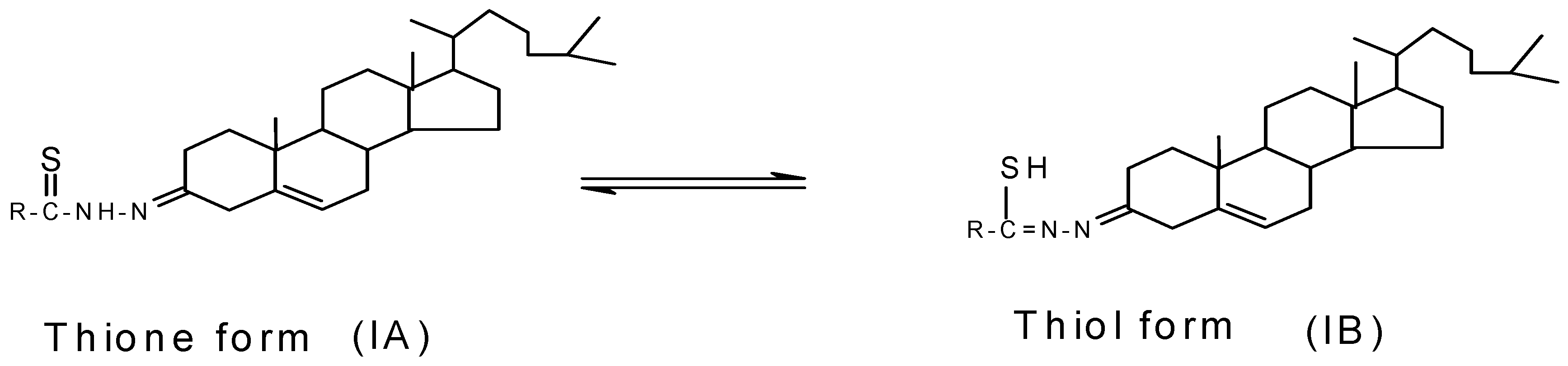
Molecules | Free Full-Text | Palladium(II) Complexes of NS Donor Ligands Derived from Steroidal Thiosemicarbazones as Antibacterial Agents

Effects of two different thiol ligands to form a mixed monolayer ligand... | Download Scientific Diagram

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -
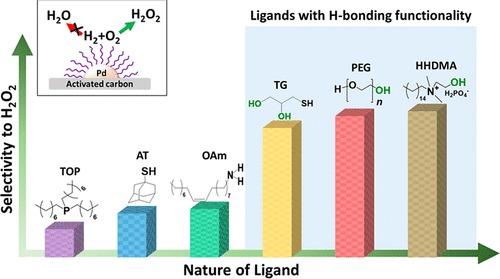
Tunable Catalytic Performance of Palladium Nanoparticles for H2O2 Direct Synthesis via Surface-Bound Ligands - ACS Catal. - X-MOL

Alkyne Hydrothiolation Catalyzed by a Dichlorobis(aminophosphine) Complex of Palladium: Selective Formation of cis‐Configured Vinyl Thioethers - Gerber - 2012 - Chemistry – A European Journal - Wiley Online Library

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
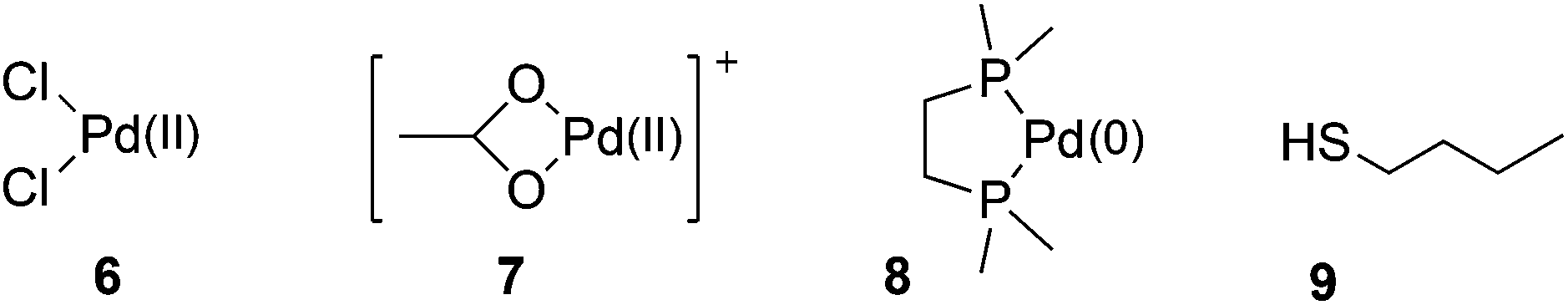
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B
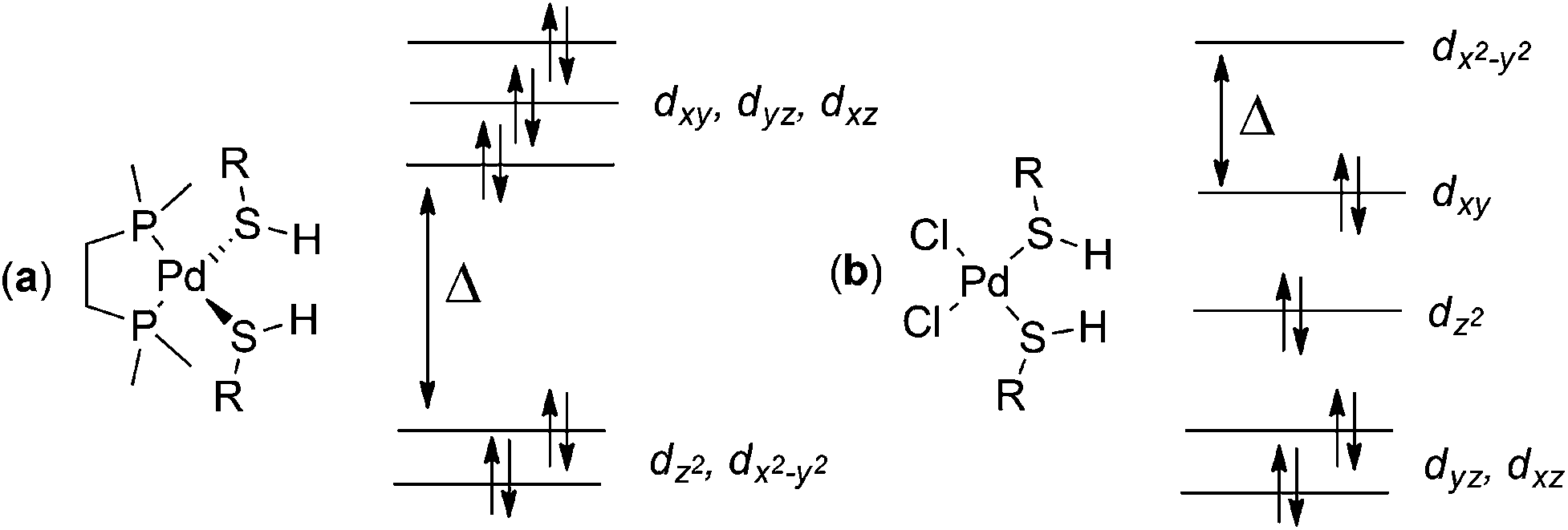
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B
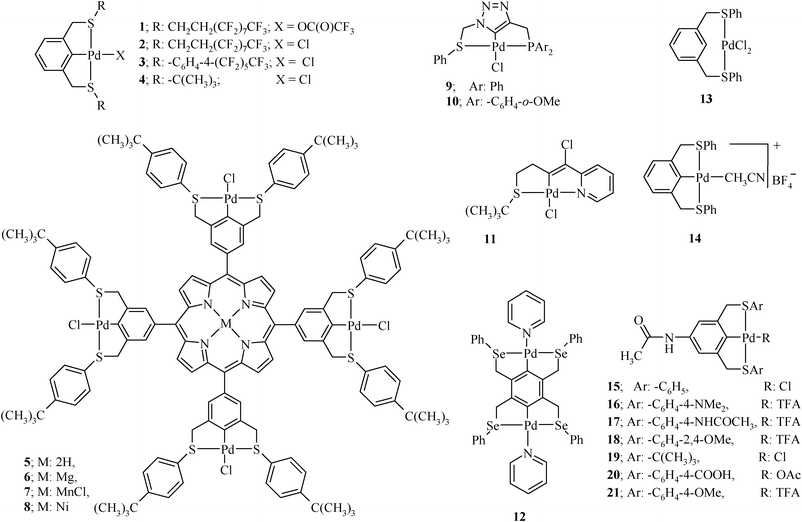
Organochalcogen ligands and their palladium( ii ) complexes: Synthesis to catalytic activity for Heck coupling - RSC Advances (RSC Publishing) DOI:10.1039/C2RA20508D
Synthesis of Alkanethiolate-Capped Metal Nanoparticles Using Alkyl Thiosulfate Ligand Precursors: A Method to Generate Promising
Synthesis of Alkanethiolate-Capped Metal Nanoparticles Using Alkyl Thiosulfate Ligand Precursors: A Method to Generate Promising
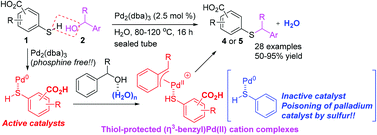
Mercaptobenzoic acid-palladium(0) complexes as active catalysts for S-benzylation with benzylic alcohols via (η3-benzyl)palladium(ii) cations in water - Organic & Biomolecular Chemistry (RSC Publishing)
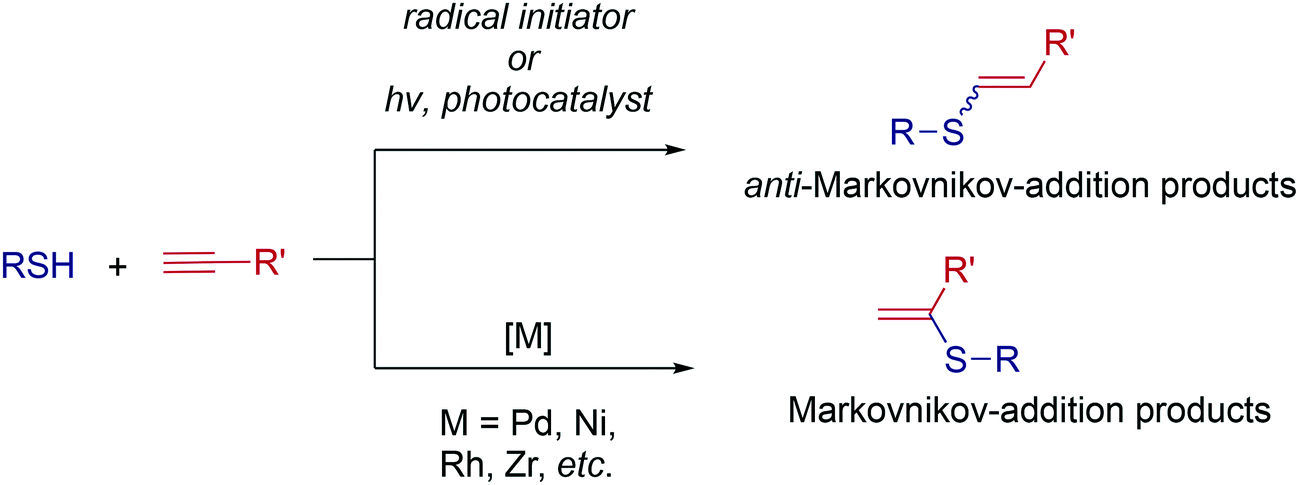
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand - Catalysis Science & Technology (RSC Publishing)