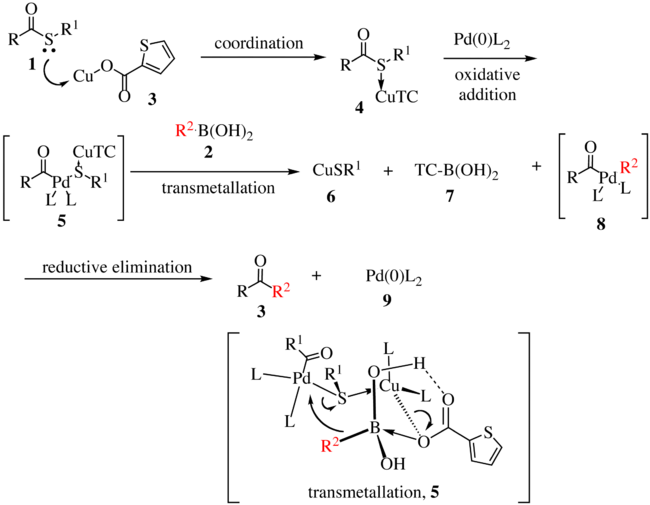
Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
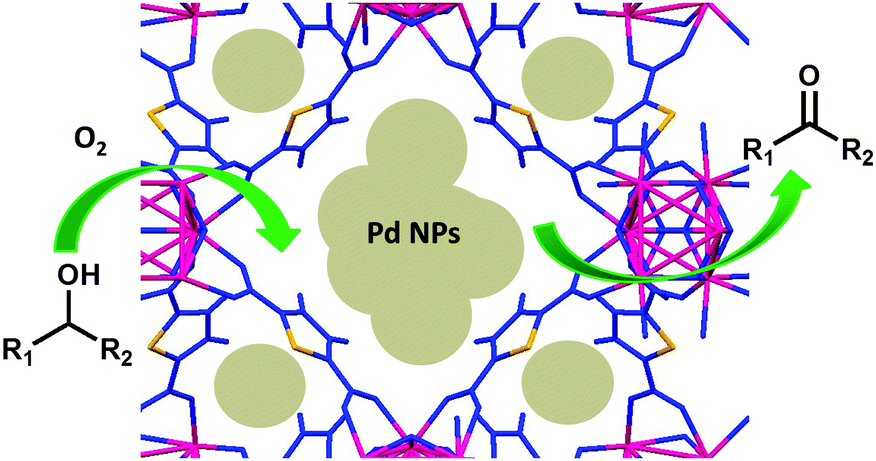
Improved activity of palladium nanoparticles using a sulfur-containing metal–organic framework as an efficient catalyst for selective aerobic oxidation in water - New Journal of Chemistry (RSC Publishing)

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Direct palladium-mediated on-resin disulfide formation from Allocam protected peptides. - Abstract - Europe PMC

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Metal‐Free Photocatalytic Aerobic Oxidation of Thiols to Disulfides in Batch and Continuous‐Flow - Talla - 2015 - Advanced Synthesis & Catalysis - Wiley Online Library
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand† - Catal. Sci. Technol. - X-MOL
![Amine-Functionalized Graphene Oxide-Stabilized Pd Nanoparticles ([email protected]): A Novel and Efficient Catalyst for the Suzuki and Carbonylative Suzuki–Miyaura Coupling Reactions - ACS Omega - X-MOL Amine-Functionalized Graphene Oxide-Stabilized Pd Nanoparticles ([email protected]): A Novel and Efficient Catalyst for the Suzuki and Carbonylative Suzuki–Miyaura Coupling Reactions - ACS Omega - X-MOL](https://xpic.x-mol.com/20190110%2F10.1021_acsomega.8b03023.jpg)
Amine-Functionalized Graphene Oxide-Stabilized Pd Nanoparticles ([email protected]): A Novel and Efficient Catalyst for the Suzuki and Carbonylative Suzuki–Miyaura Coupling Reactions - ACS Omega - X-MOL

Optimising surface d charge of AuPd nanoalloy catalysts for enhanced catalytic activity | Nature Communications
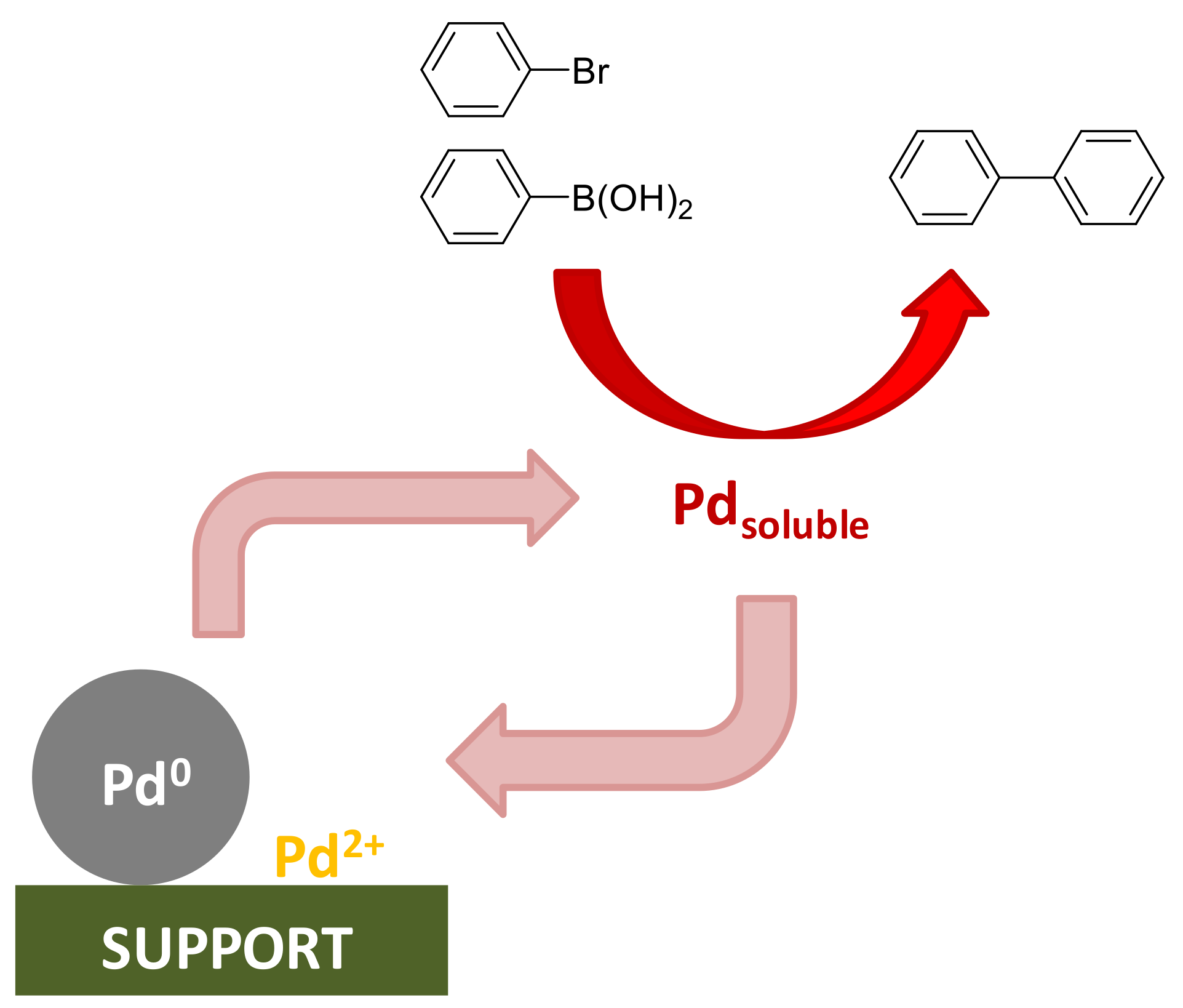
Materials | Free Full-Text | Thiol-Functionalized Ethylene Periodic Mesoporous Organosilica as an Efficient Scavenger for Palladium: Confirming the Homogeneous Character of the Suzuki Reaction
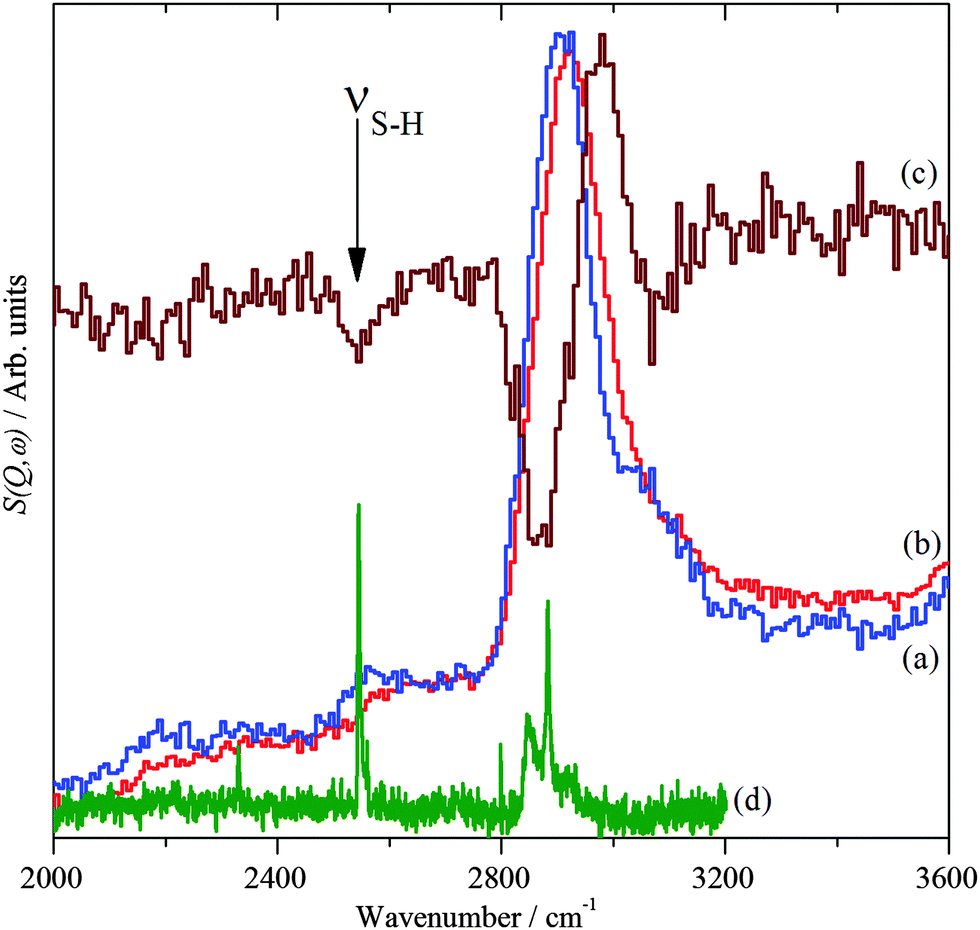
The adsorbed state of a thiol on palladium nanoparticles - Physical Chemistry Chemical Physics (RSC Publishing)

KR20140016251A - Palladium-copper catalysts for the homogeneous selective oxidation of thiol groups - Google Patents